Two Covid-19 vaccines approved

The Drug Controller General of India (DCGI), the country’s national drug regulator, announced on Sunday (January 3) that the Central Drugs Standard Control Organisation (CDSCO) has decided to accept the recommendations of its Subject Expert Committee (SEC), and approved the Covid-19 vaccines of both Serum Institute of India and Bharat Biotech for restricted use in the country. ?
Government will procure vaccines
Serum Institute of India (SII), has manufactured Covishield, the Indian variant of the AZD1222 vaccine developed by Oxford University and AstraZeneca, and already stockpiled some 80 million doses. As such, the rollout can begin fairly quickly.
The other vaccine that has got emergency use authorisation, Covaxin, manufactured by Hyderabad-based Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR), could take a few days or weeks to be
Process of administering will start
In the United States and United Kingdom, the first shots were administered within 1-2 days of the Pfizer-BioNTech and Moderna vaccines receiving regulatory approval. In India too, the process is likely to be very fast. While no concrete timelines have been announced it, it can be reasonably expected that the mass vaccination programme will begin within a week — perhaps by the coming weekend.
The first recipients of the vaccine
First, the entire vaccination drive will be voluntary. The government has already announced that first in line will be 30 million (3 crore) workers in the forefront of India’s battle against the novel coronavirus, including 1 crore healthcare workers and 2 crore frontline workers. Health Minister Dr Harsh Vardhan announced on Saturday (January 2) that the vaccine will be administered to them for free. Also receiving the vaccine in the first phase will be a third priority group – consisting of some 27 crore persons above age 50, and persons below age 50, but with associated comorbidities.
“In 1st phase of #COVID19Vaccination, free #vaccine shall be provided across the nation to most prioritised beneficiaries that incl[ude] 1 crore healthcare & 2 crore frontline workers. Details of how further 27 cr[ore] priority beneficiaries are to be vaccinated until July are being finalised,” Dr Harsh Vardhan tweeted on January 2 .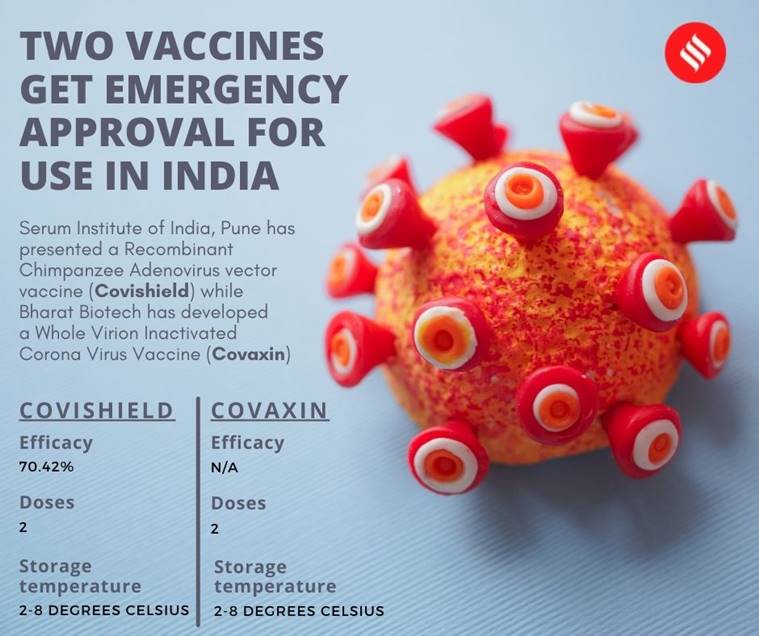 Two vaccines get emergency approval for use in India
Two vaccines get emergency approval for use in India
What about the rest of the population?
The government aims to complete the first phase of vaccinations by August 2021. The timelines for the rest of the population are not known as yet. However, it is not as though the rest of the population will have to wait until the first phase of vaccination is complete. Other groups will begin to be inoculated simultaneously after some weeks or months.
Critical here will be the pace of the vaccination, and the availability of the required doses. SII has said it is ramping up production at its facilities. Also, a whole lot of other vaccines — apart from Covishield and Covaxin — are likely to be approved for use in India in the coming weeks. These include the vaccines from Pfizer and Moderna, the Russian Sputnik-V, and Zydus-Cadila’s ZyCoV-D candidate
Preparations for the vaccination
Preparations have been on for weeks for what will be India’s widest and most ambitious vaccination drive ever. Two rounds of mock drills have already been held — the first in four states on December 28-29, and the second on January 2, covering 285 session sites in 125 districts in all states around the country.
Some 96,000 vaccinators have been trained, including 2,360 in a National Training of Trainers, and over 57,000 in district-level training carried out in 719 districts. More than 75 lakh beneficiaries have been registered on the Co-WIN platform, which will provide real-time information on vaccine stocks, their storage temperature, and individual beneficiaries of the shots, Dr Harsh Vardhan said on Saturday.
The country’s cold chain infrastructure has been sufficiently upgraded to ensure last-mile delivery, and adequate supplies of syringes and other logistics have been arranged, he said.Also in Explained |When India-UK flights resume on Jan 8, what will be the guidelines? During a vaccination dry run in Kolkata
During a vaccination dry run in Kolkata
If you have recovered from Covid, will you be vaccinated?
Yes. The centre has said that it is advisable to receive a complete schedule of COVID vaccine irrespective of the past history of infection with COVID-19. “This will help in developing a strong immune response against the disease,” the centre has said.
How will the government identify those above 50 years and above? Is there a sub priority group for those over the age of 50 years?
The government has said that the latest electoral roll for the Lok Sabha and Legislative Assembly election will be used to identify the population aged 50 years or more.
The priority group of above 50 years is further subdivided into those above 60 years of age and those between 50 to 60 years of age for the phasing of roll out based on “pandemic situation and vaccine availability”.
How many doses will be administered? When do we potentially develop immunity?
According to the Health Ministry, two doses of vaccine, 28 days apart, need to be taken by an individual to complete the vaccination schedule. Protective levels of antibodies are generally developed two weeks after receiving the 2nd dose of COVID-19 vaccine, the health ministry has said.